Dye Analysis Methods
Below are three examples of dye analysis and the benefits
and problems using them are discussed.
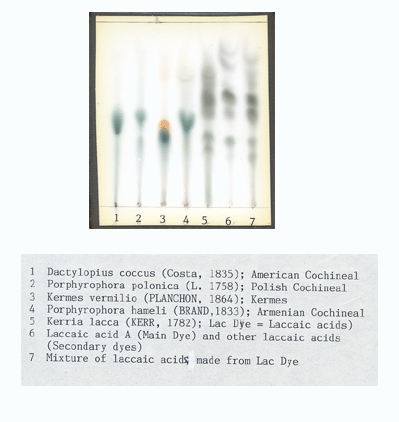
This is a Thin Layer Chromatography plate with dyes from insects on it. Notice the starting point is at the bottom of the plate and the dye migrates up the plate leaving "dots" or elliptical spots.
This method has the advantage of showing how one unknown sample and a standard compares. There is some separation as the emerging dots represent dyes and dye components. It is difficult to determine which dot in a single column is which dye or dye component unless it is next to a standard. Here is a collection of dye insect standards (courtesy of a master of TLC, Dr. Helmut Schweppe).
Thin
Layer Chromatography (
TLC )
No
method of dye analysis is usable without dye standards. Identification
of dyes in a mixture is essential because dyes were frequently mixed
when used on yarns or several different yarns mixed in a textile. The
more dyes and their standards you can compare the unknown sample with-
the more potential discovery from unknown samples is possible.
![]()
The disadvantage is that you pretty much have to know what the dye is before using this test because you have to use a standard on the same plate with the unknown sample. Different dyes tested require different solvent baths with the plates to cause the samples to migrate, so you have to have a good idea what the dye is or just test for, as in this case, red insect dyes.
Another disadvantage is you have to have on hand standards that are difficult to obtain. I use 69 pre-1900 synthetic dyes along with an extensive collection of natural dyes. Imagine the number of plates needed to identify a synthetic dye from standards that could be mixed with who knows which natural dyes... Dr. Schweppe used and taught me how to use Chemical Spectrometry (colors change in solution while a fairly large yarn sample's dyes react in a test tube to changes in the chemistry) to generate a hypothesis about what the dye was and then test the hypothesis with TLC. Large several gram yarn samples are needed to do this...
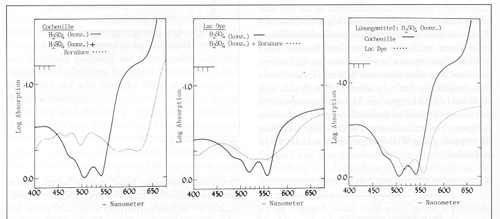
Spectrometric dye analysis results are shown here of cochineal and lac. The spectral curves vary with the solvents or acids used.
This method has the advantage of showing you a sample that can be compared with a curve of a standard.
The disadvantage of this method is the dyes can not be physically separated out from a mixture and identified individually.
Mixtures need to be analyzed as a blend of dyes in the mixture. If you have two different dyes on a yarn that you want to analyze you need to make standards of all the variations the dyes. You need to mix various ratios to provide spectral standards to compare to unknowns. Software can help do this better today, but mixtures of unknowns are a problem for this method.
A textile that has various yarns dyed with a synthetic dye and another yarn dyed with cochineal twisted together to make one red color- means you better be able to separate the dyes in the sample tested.
Spectrophotometric
Spectral curves of Cochineal on the left and Lac in the middle with both Cochineal and Lac OVERLAID- one dye test result physically on top of the other.
No spectral curves of a mixture of Cochineal and Lac is shown.
*** No SEPARATION of dyes in a mixture are possible using this method.***
Test results of a mixture of dyes will have a different curve than either dye in the mixture alone and the spectral curves varies with various ratios of dyes in the mixture.
Cochineal below left and Lac in center
below. The two curves each have differernt acids
in the back ground as the lighter line for comparison.
Curves at right below are Cochineal overlaid with Lac- NOT a Mixture.
The spectral curve
of a mixture could be very confusing as the lower part of the curve
in both Cochineal and Lac is very similar. This
becomes more problematic when the pH of a solution is not the same as
the standard solution. When we imagine a spectral curve for a
mixture of Lack and Cochineal and the mixture has different amounts
of Lac and Cochineal in the sample because yarn extractions are never
the same ration of dyes in a mixture. Oringally, dye masters frequently
added yellow dyes and we find contaminations from dyes on neighboring
yarns in the same textile. All these variables
cause changes in the spectral curve with this method.
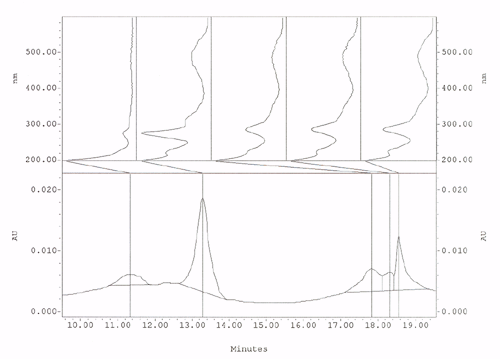
This is a chromatograph produced by HPLC in the bottom half illustrated
as peaks emerging over time. The top half is the spectral absorbency curve
between 200nm and 600nm for each peak.
The advantage is this method separates each dye and dye component clearly for spectral analysis. Statistical dye component analysis is achieved by using each peak area of all the dye components to calculate the total percentage of dyes found and then the total divided into each peak area to give the percentage of each dye and dye component present. State of the art qualitative/kind of dye and quantitative/amount of dye- analysis from a 2mg to 4mg sample.
High Pressure Liquid Chromatography ( HPLC )
Results
of testing a mixture of Cochineal and Lac.
Dyes
are clearly SEPARATED out of a mixture of Cochineal and Lac:
Cochineal is the large peak on the left and Lac dye components are the
three peaks on the right. The spectral curves for each peak are displayed
above the peaks.
![]()
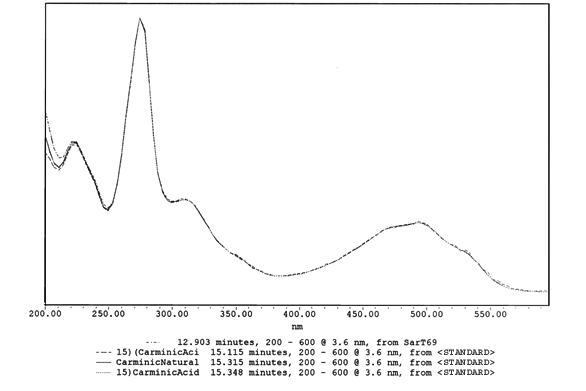
This is one peak's spectral curve Report produced from a Photodiode Array detector ( PDA ). The computer has compared the spectral curve reports in the computerized library to produce this report. Carminic acid is the main dye component for cochineal and the same spectral curve comparisons are made for lac or any other dye spectral curves found.
HPLC with a PDA detector is the clearest, easiest, and mathematically based dye analysis method available with today's technology.
We use over 450 dye spectral curves when
we compare each unknown sample to the standards within the computer library.
For a detailed outline of complete textile analysis and
certification please see methods below.
![]()
![]()
Spectral Report of Standards and Uknown Sample
This
is the 1st sepctral absorbance curve shown above for cochineal, with standards
from Cochineal insects overlayed with unknown samples from the SAR-T69
textile.
*The big advantage to this method is the spectral curve remains constant*
as does the pH of the sample, so standard spectral curves can be consistently
and reliably compared to unknown sample spectral curves. These spectral
curves can be analyzed separately from the chromatograph shown above.
Computerized libraries of standards and their spectras
are used to create the spectral report shown below.
This is the same spectral curve as the one on the left above identified
from cochineal.
Call
Casey Reed 505-344-8492